
Four Ways to Increase Clinical Trial Diversity
The FDA has heightened its focus on increasing racial and ethnic diversity in clinical trials. According to a recent study by Nature Reviews Disease Primers, “in a 2020 analysis of the global participation in clinical trials, the FDA highlighted the vast difference between the enrolled participants and the global population. Of 292,537 participants in clinical trials globally, 76% were white, 11% were Asian and only 7% were Black. In comparison, the global population (~7.8 billion) is distributed with ~60% of the population in Asia, ~16% in Africa, ~10% in Europe and ~8% in Latin America (World Population Review).”
The Role of Drivers of Health
Life sciences organizations are increasingly aware of the powerful role drivers of health (DOH) play in whole-person care. DOH are non-clinical contributors to whole-person health. Such behavioral, personal, lifestyle, socio-economic, and environmental factors are responsible for around 80 percent of an individual’s overall health status. However, it’s often a challenge for life sciences organizations to identify DOH needs for the communities they serve and to successfully factor them into research and development efforts. Clinical trial diversity, in particular, has remained both a pressing challenge and a key priority.
Interview with Dr. Rodrigo Burgos on Clinical Trial Diversity
We interviewed industry expert Dr. Rodrigo Burgos, Clinical Assistant Professor and HIV PGY-2 Residency Co-Director at the University of Illinois Chicago, to share his thoughts on the evolving matter of clinical trial diversity. As an HIV pharmacotherapy specialist and pharmacy residency director, Dr. Burgos is committed to expanding opportunities to support pharmacists who serve minority communities.
What are some ways that pharma organizations can expand clinical trials to be more diverse?
When thinking about ways for pharma to expand diversity in clinical trials, the first thing to note is that understanding diversity, equity, and inclusion (DEI) in clinical trials is complex. There is no single root-cause, nor a single solution. Expanding clinical trial diversity is a long-term commitment and ongoing effort that must come from all of us.
That said, there are some guidelines that many experts in this space recommend:
Four Ways to Increase Clinical Trial Diversity
1. Understand regulatory evolution: Since around 2006, U.S. legislation such as the Prescription Drug User Fee Act (PDUFA), CURES Act, DEPICT Act (Diverse and Equitable Participation in Clinical Trials) and other laws have come into effect that incorporate aspects of diversity and inclusion in therapy development. (See Figure 1 below.) For instance, the FDA Guidance on Collection of Race and Ethnicity Data in Clinical Trials; Evaluation and Reporting of Age, Race, Ethnicity Data provides helpful guidance on this topic.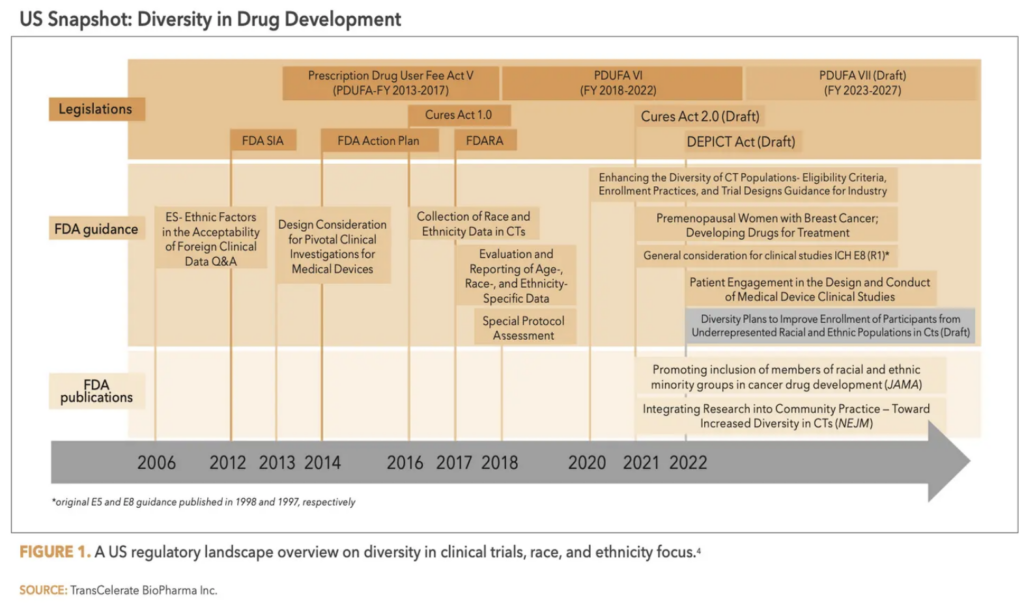
2. Incorporate a DEI team and sponsors: Consider implementing a DEI team that keeps track of the regulatory landscape and assists with DEI implementation in clinical trials. Sponsors (a person, company, institution, group, or organization that oversees or pays for a clinical trial and collects and analyzes the data) can also work with sites on how to best approach their diverse patient populations. Having a dedicated support team for this work can go a long way in furthering clinical trial diversity.
3. Engage in collaborative data-sharing relationships: The FDA requests sponsors define their enrollment goals for diverse populations as early as possible. Goals are established based on gold-standards or benchmarks, which are defined based on epidemiological data. Many disease states have little to no epidemiological data to inform their gold standards, which can hinder research efforts in the long term.
4. Take a human-centered design approach: Putting real people at the center of the trial development process is critical. We must engage with communities and their leaders to understand the best ways to reach, educate, and communicate with prospective patients and study participants. Consider key areas such as:
- Communication methods: What is the best way to reach this audience (social media, advertising, community events, etc.)?
- Messaging: Is the messaging about the trial culturally sensitive? Does the community we’re trying to reach understand the key message?
- Reading comprehension: Is the reading level appropriate for this audience?
- Language: Are we using the same language as the community we’re trying to reach?
- Imagery: Are the images in our outreach culturally sensitive and relevant?
Advancing Whole-Person Care, Health Equity, and Organizational Performance
Every healthcare organization is more aware today of how disparities in health equity and DOH influence health outcomes, whole-person health, and overall healthcare costs.
Life sciences organizations can take a leading role in addressing these disparities and improving whole-person health by incorporating DOH insights into their business processes and by coordinating services with providers, payers, community partners, advocacy and support organizations, government agencies, and patients. Specifically, they can leverage data-driven insights to support strategic initiatives that advance adherence and improve outcomes.
By addressing the drivers of health, life sciences organizations can help build the future infrastructure of care and meet the challenge of health equity through targeted and effective investments.
Read the full Q&A report to learn more about how life sciences organizations can improve clinical trial diversity.
About Unite Us
Unite Us is the nation’s leading software company bringing sectors together to improve the health and well-being of communities. We drive the collaboration to identify, deliver, and pay for services that impact whole-person health. Through Unite Us’ national network and software, community-based organizations, government agencies, and healthcare organizations are all connected to better collaborate to meet the needs of the individuals in their communities.



